The tablet coating process transforms raw tablets into sophisticated products that balance efficacy, stability, and patient appeal. Film coating, a critical step, encapsulates tablets with a thin, uniform layer to protect active pharmaceutical ingredients (APIs), control drug release, enhance aesthetics, and improve patient compliance.
I. Understanding Film Coated Tablets
A film coated tablet is a solid oral dosage form enveloped in a thin, polymer-based layer that serves multiple functions, from safeguarding the API to enhancing swallowability. Unlike uncoated tablets, which are vulnerable to environmental degradation, film coating provides a protective barrier, improves visual appeal through color and gloss, and can modify drug release profiles. This process is not merely cosmetic; it’s a technologically advanced operation that ensures tablets meet stringent regulatory standards while addressing patient needs. The film coating process for tablets is widely adopted due to its versatility, supporting immediate-release (IR), delayed-release, or sustained-release formulations tailored to specific therapeutic goals.

II. The Multifaceted Purpose of Tablet Coating
The tablet coating process is indispensable in pharmaceutical manufacturing, offering benefits that span production, drug performance, and patient experience. Here’s why it matters:
Manufacturing and Storage Stability:
- Film coating shields APIs from environmental stressors like moisture, light, and oxygen, which can trigger hydrolysis, oxidation, or photodegradation. For moisture-sensitive drugs like ranitidine hydrochloride, coatings with polymers like polyvinyl alcohol (PVA) or Eudragit enhance stability, extending shelf life and maintaining efficacy during storage.
- By preventing physical changes like swelling or cracking, coatings ensure tablets remain intact within packaging, preserving product integrity.
Controlled Drug Release Patterns:
- Coatings enable precise control over the site, rate, and timing of API release. Immediate-release coatings dissolve quickly in the stomach, while enteric coatings, designed for drugs like proton pump inhibitors (e.g., omeprazole), delay release until the small intestine to protect APIs from gastric acid or prevent gastric irritation.
- Sustained-release coatings, using water-insoluble polymers like ethyl cellulose, prolong drug release, reducing dosing frequency and improving therapeutic outcomes.
Enhanced Patient Compliance:
- Film coating improves swallowability by creating a smooth, glossy surface, making tablets easier to ingest, especially for pediatric and geriatric populations. Taste-masking properties hide bitter or unpleasant flavors, encouraging adherence.
- Color-coded coatings aid identification, reducing medication errors, and clear, legible markings ensure traceability, meeting regulatory demands for safety and compliance.
Aesthetic and Functional Quality:
- A uniform, defect-free coating enhances tablet appearance, reinforcing brand identity and consumer trust. It also minimizes defects like bridging, cracking, or orange-peel roughness, ensuring compliance with compendial standards.
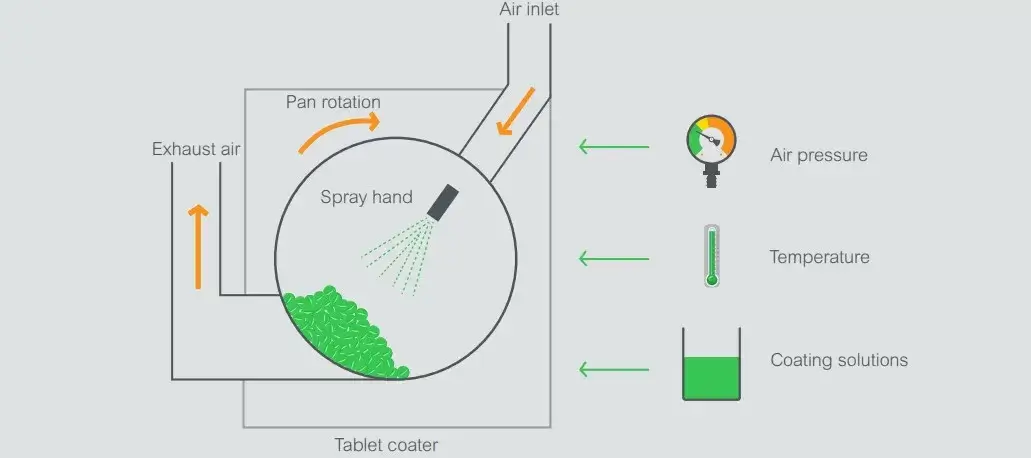
Tablet Coating Process
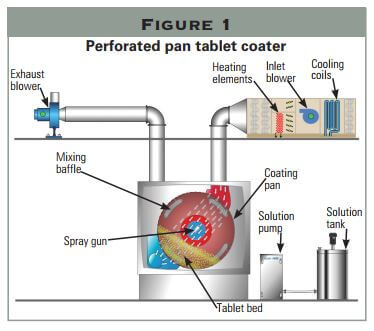
Pan Tablet Coater
III. How the Pharmaceutical Film Coating Process Works
The film coating process for tablets is a sophisticated, multi-step operation that demands precision to achieve consistent, high-quality results. Here’s a detailed breakdown:
Formulation Preparation:
- The coating formulation blends film-forming polymers (e.g., hydroxypropyl methylcellulose [HPMC], Eudragit), plasticizers, pigments, and additives to achieve desired properties like color, gloss, and moisture resistance. The choice of polymer significantly influences viscosity, which affects sprayability and film quality.
Coating Solution Preparation:
- Pre-formulated powder is mixed with water or an organic solvent, typically requiring 45 minutes to create a homogeneous solution. The viscometer for coatings is critical here, as viscosity directly impacts droplet formation and film uniformity. High-viscosity solutions risk lump formation, while low viscosity ensures faster preparation and better surface quality.
Application of Coating:
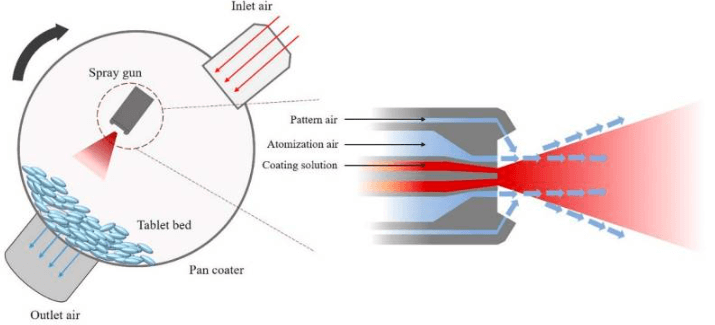
- Loading: Tablets are placed in a coating machine, such as a pan coater or fluidized bed coater, where they rotate to ensure even exposure to the coating solution.
- Spraying: The coating solution is atomized through a spray nozzle, with atomization and pattern air controlling droplet size and distribution. A balanced air-to-spray ratio (ideally 1:1) ensures smaller droplets and uniform coverage.
- Drying: Hot airflow evaporates the solvent, forming a smooth, continuous film. Controlled drying prevents over-drying (causing rough surfaces) or under-drying (leading to twinning or agglomeration).
Quality Control:
- Rigorous monitoring of coating thickness, uniformity, color, and texture ensures compliance with good manufacturing practices (GMPs). Inline measurements, particularly of viscosity and density, are vital to prevent defects like bridging, cracking, or peeling, as highlighted in process control discussions.
What Are the Best Tablet Coating Solutions?
Choosing the right coating solution depends on the drug’s properties, therapeutic goals, and manufacturing constraints. Two primary methods dominate:
Organic Solvent Film Coating:
- Ideal for moisture-sensitive APIs, organic solvent coatings use polymers like cellulose acetate phthalate to provide robust moisture barriers, preventing hydrolysis. However, they are costlier, pose safety risks due to flammability, and have environmental drawbacks, requiring specialized handling and disposal.
Aqueous Film Coating:
- The preferred choice for most applications, aqueous coatings use water-soluble polymers like HPMC or PVA, offering scalability, safety, and reduced environmental impact. Advanced formulations enhance efficiency across equipment types, making them cost-effective and compliant with regulatory trends, such as restrictions on titanium dioxide (TiO2).
IV. Pharmaceutical Applications of Film Coating
The tablet coating process serves diverse applications, each addressing specific pharmaceutical challenges:
- Modified Drug Release:
Delayed Drug Release:
- Enteric coatings protect acid-labile drugs (e.g., esomeprazole) or reduce gastric irritation (e.g., pantoprazole) by dissolving in the small intestine’s basic pH. Dual delayed-release formulations, like dexlansoprazole, combine granules with different pH-dependent dissolution profiles for extended absorption.
- Colon-targeted coatings, using pH-dependent or enzymatically degradable polymers, treat conditions like Crohn’s disease or improve peptide bioavailability. Technologies like ColoPulse integrate pH and bacterial triggers for precise delivery.
- Chronotherapeutic coatings align drug release with circadian rhythms, as seen in enteric-coated bilayer tablets of telmisartan and pravastatin, optimizing treatment for hypertension and cholesterol synthesis.
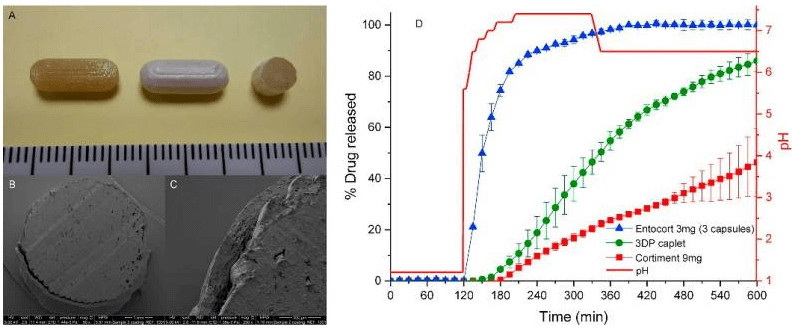
Figure 1
The coated caplet was resistant to acidic conditions and exhibited a pH-dependent drug release profile. It started drug release after 1 h in the small intestinal condition and then continued drug release in a sustained manner throughout the conditions of distal intestine and colon
Sustained Drug Release:
- Water-insoluble polymers like ethyl cellulose or polymethacrylates create sustained-release profiles, reducing dosing frequency for drugs like venlafaxine. Osmotic pump systems, coated with cellulose acetate, control release through fluid penetration and orifice size, as demonstrated in eperisone hydrochloride formulations.
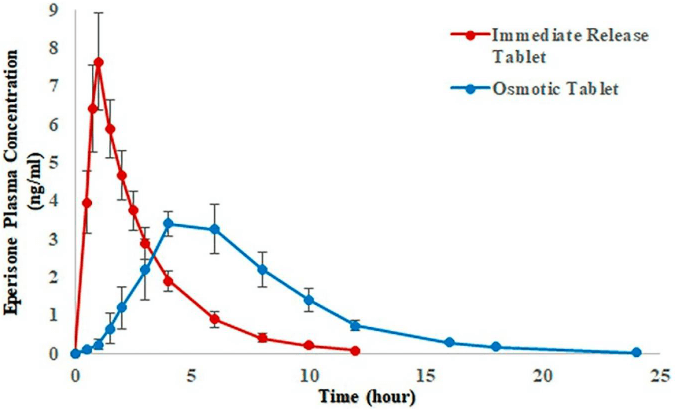
Figure 2
Mean plasma concentration versus time profiles obtained after administration of Eperisone 150 mg CR (controlled release) osmotic and immediate release tablets.
Improved Drug Stability:
- Film coating protects moisture-sensitive (e.g., ranitidine) or light-sensitive drugs (e.g., nifedipine) using hydrophobic polymers, lipids, or opacifiers. Combining polymers like HPMC with suberin fatty acids enhances water vapor barriers, ensuring long-term stability.
Taste Masking:
- Essential for pediatric and geriatric compliance, taste-masking coatings use polymers like ethylcellulose or hypromellose to prevent bitter drug release in the oral cavity. Optimized ratios of water-soluble and insoluble polymers balance taste masking with bioavailability.
Active Film Coating:
- This innovative approach incorporates APIs into the coating layer, enabling fixed-dose combinations (e.g., metformin and glimepiride) or improved stability for drugs like peliglitazar. Challenges include achieving uniform API distribution and precise end-point control, requiring robust process monitoring.
V. Process Challenges in Tablet Coating
The film coating process for tablets is dynamic, with spraying, coating distribution, and drying occurring simultaneously. Key challenges include:
- Solvent Loss and Viscosity Changes: Solvent evaporation increases viscosity, affecting droplet formation and film quality. Periodic thinner additions are necessary to maintain optimal viscosity.
- Defect Formation: Issues like bridging, cracking, orange-peel roughness, or twinning arise from improper process parameters, such as unbalanced spray air, incorrect droplet size, or inadequate drying.
- Traditional Measurement Limitations: Offline tools like efflux cups or laboratory viscometers are inaccurate and time-consuming, failing to capture in-process conditions like temperature, shear rate, or flow dynamics.
- Regulatory Compliance: Strict standards demand consistent color, legibility, and defect-free coatings, necessitating real-time control to meet GMPs and traceability requirements.
VI. Process Parameters and Factors Affecting Film Coating Quality
Achieving a uniform, high-quality coating requires meticulous control of process parameters, as outlined below:
Spray Air Flow Rate:
- Atomization and pattern air disintegrate the coating solution into droplets. A 1:1 ratio minimizes droplet size, enhancing coating uniformity and efficiency. Imbalanced air flows lead to uneven deposition and defects.
Spray Rate:
- Higher spray rates increase droplet size and reduce velocity, impacting coating quality. The atomization air-to-spray rate ratio is critical for maintaining consistent droplet size and drying capacity.
Inlet and Outlet Air:
- Inlet air temperature and humidity affect drying efficiency. Over-drying causes rough surfaces, while under-drying leads to agglomeration. Outlet air temperature, typically 2–3°C above tablet bed temperature, guides drying adjustments.
Droplet Size:
- Smaller droplets, achieved through balanced air ratios and lower viscosity, ensure a homogenous film. Large droplets increase surface roughness, compromising quality.
Solid Content and Viscosity:
- High solid content accelerates weight gain but increases viscosity, complicating spraying. Heating the solution or optimizing polymer content lowers viscosity, improving processability and reducing costs.
Gun-to-Bed Distance:
- Optimal distance ensures droplets reach the tablet surface without premature drying or excessive wetness. Too far, and droplets dry mid-air, causing roughness; too close, and wet surfaces lead to twinning.
Curing Time:
- Post-coating curing (1–several hours) removes residual solvent and hardens the film, affecting dissolution profiles. Inadequate curing risks incomplete polymer coalescence.
Pan Speed and Tablet Movement:
- Proper pan rotation ensures tablets cycle through the spray zone with varying orientations, promoting uniform coating. Slow speeds cause uneven coverage, while excessive speeds may damage tablets.
Coating Solution Composition:
- The choice of polymers, pigments, and plasticizers influences viscosity, surface tension, and film quality. Water-soluble polymers increase viscosity, requiring careful formulation to avoid defects.
VII. The Role of the Lonnmeter Inline Coating Viscometer
The Lonnmeter Inline Coating Viscometer revolutionizes pharmaceutical printing and coating by providing real-time viscosity monitoring, addressing the limitations of traditional tools. This advanced coating viscometer measures viscosity directly in the process stream, accounting for variables like temperature, shear rate, and flow conditions.
Advantages of the Lonnmeter Inline Coating Viscometer
- Real-Time Precision: Continuously monitors viscosity changes from a baseline, enabling immediate adjustments to solvent levels or temperature, ensuring consistent droplet formation and film quality.
- Defect Reduction: Maintains optimal viscosity to minimize issues like bridging, cracking, or orange-peel roughness, ensuring compliance with finished product specifications.
- Cost Efficiency: Reduces pigment and solvent use by optimizing solid content, lowering material costs and waste, and shortening processing times.
- Environmental Benefits: Minimizes resource consumption, aligning with sustainable manufacturing practices.
- Enhanced Compliance: Ensures consistent color density and legible markings, critical for regulatory traceability and patient safety.
- Operator Efficiency: Automates viscosity control, freeing operators to focus on other tasks, unlike manual methods that are time-consuming and inconsistent.
- Scalability: Adapts to various coating equipment (pan coaters, fluidized bed coaters) and formulations, supporting both aqueous and organic solvent processes.
Mastering the film coating process for tablets requires precision, advanced technology, and a deep understanding of process parameters. The Lonnmeter Inline Coating Viscometer empowers manufacturers to overcome challenges, optimize quality, and achieve compliance with ease. Request a quote today to discover how this cutting-edge viscometer for coatings can enhance efficiency, reduce costs, and deliver superior tablets that meet patient and regulatory demands.
Post time: Aug-22-2025









